What Does Electronic Data Capture Mean
Once youre ready to pull the data out of the system there are easily accessible exports to extract patient data. See also automatic data capture.

What Is Data And Why Is It Important Import Io
EDC process happens after authorization.

What does electronic data capture mean. Using systems to collect clinical trial data in electronic form as opposed to paper form. Define Electronic Data Capture. Possible ELECTRONICDATACAPTURE meaning as an acronym abbreviation shorthand or slang term vary from category to category.
Level up your career in data capturing structural assessments and lifecycle analysis. Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. Electronic Data Capture for clinical investigations and post market clinical follow-up.
Electronic Data Capture EDC What Does Electronic Data Capture EDC Mean. An EDC system. Annonce Electronic Data Capture thats tailor-made for MedTech studies.
The EDC initiates the withdraw of the electronic bank draft while the authorization confirms the accounts ability to. Electronic Data Capture EDC is the medical abbreviation is admittedly a fairly generic sounding term but in the clinical trials field it actually means something fairly specific. Electronic data capture EDC is the computerized collection and management of clinical trial data from patients and subjects.
Level up your career in data capturing structural assessments and lifecycle analysis. Definition - What does Electronic data capture mean. Use of point-of-sale terminal or a specialized software for online transactions for submitting and validating credit card transactions to a merchant account provider or some other credit card transaction processor.
At OpenClinica we are immersed in EDC all day every day. More generally data capturing can also refer to collecting relevant information whether sourced from paper or electronic documents. Điều hướng bài viết.
An EDC system uses technology to streamline the collection and transmission of clinical trial data from the patient to the research laboratory. OrEDC shall mean the process of collecting study data in a permanentelectronic form. We know 2 definitions for ELECTRONICDATACAPTURE abbreviation.
Data capture or electronic data capture is the process of extracting information from a document and converting it into data readable by a computer. This is the actual transfer or capture of funds that are drafted into the merchants account electronically. Please look for them carefully.
Edcsystem clinicalresearch electronicdatacapture clinicalgyanEDC- Electronic Data Capture Systems as mainstream in clinical researchThis video explains. Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. Electronic data capture là gì.
Any method of collecting information and then changing it into a form that can be read and used. Turning Documents into Data. Electronic data capture systems have become a very popular tool to use in managing data in clinical trials.
Annonce Electronic Data Capture thats tailor-made for MedTech studies. What does ELECTRONICDATACAPTURE mean. Some systems have built-in metrics reporting that offer insights into the progress of a study.
Electronic Data Capture for clinical investigations and post market clinical follow-up. For ELECTRONIC DATA CAPTURE we have found 2 definitions.

In Essence The Standard Definition Of Data Entry Is To Key Information Into The Computer In Form Of From Forms Or Non Electronic Data Entry What Is Data Data

What Is Ecoa And How Does It Improve Clinical Trial Data Quality

Quantitative Impact Study Basel Iii Monitoring European Banking Authority

We Can Resolve Your Technical Problems Or Fix And Upgrade Older Systems Were Possible Sst Tech Techn Electronic Data Systems Technology Essential Oil Mixes

The Eip Laser Turntable Plays Records Without Touching Them Decoded Magazine Turntable Hifi Audio Audio Design
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor
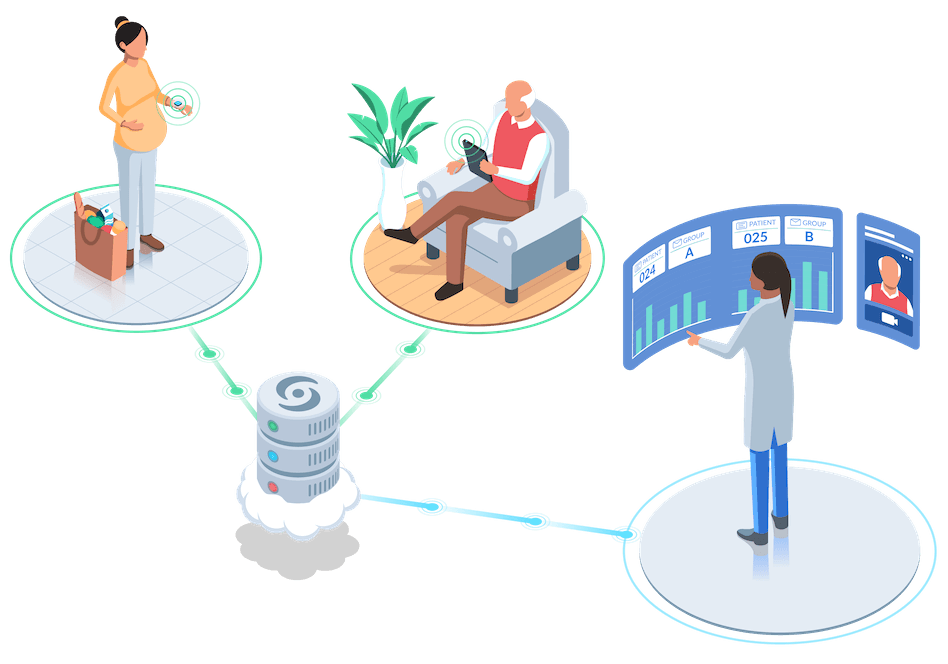
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor
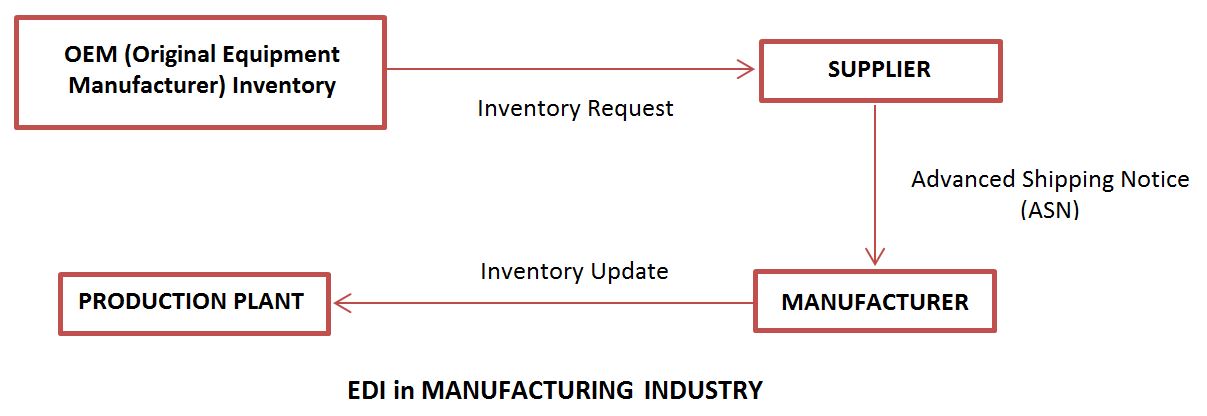
Electronic Data Interchange Edi Components Applications Advantages Bba Mantra

Openclinica Clinical Data Management Epro And Ecrf
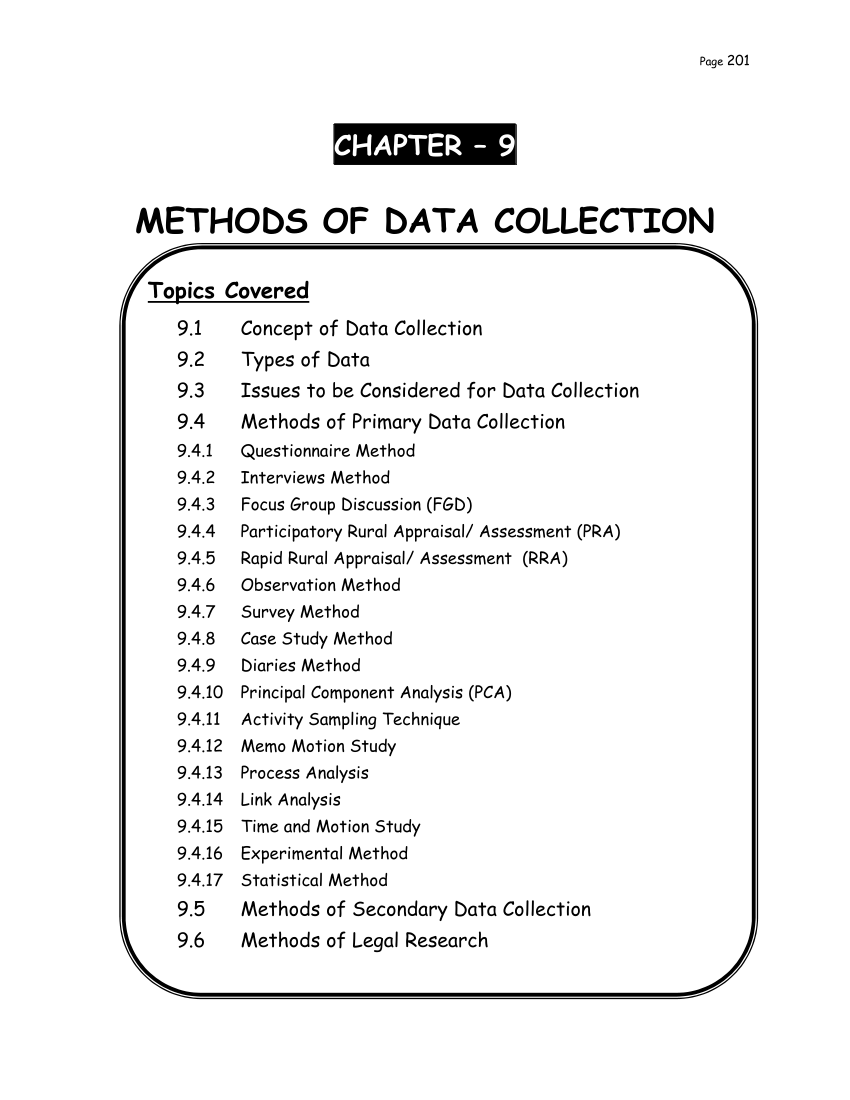
Pdf Methods Of Data Collection

Structured Data Conceptual Workflow Data Capture Conceptual Framework Data

Risk Based Monitoring Toolbox Ecrin

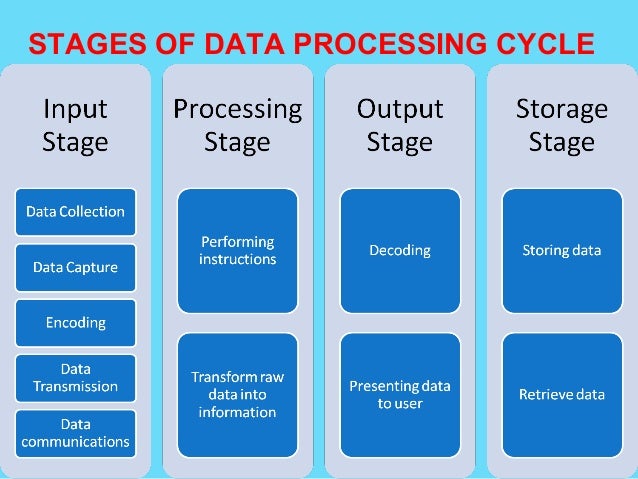
Data Processing Explained Definition Stages Use Importance
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf


Post a Comment for "What Does Electronic Data Capture Mean"