Electronic Data Capture Certification
The course will address evaluation of systems user requirements and process change and implementation of. This module covers standards for study processes concepts for regulatory compliance and electronic data capture fundamentals.
Electronic Data Capture Software Edc Clinical Trial Management Software Ecaselink
But if you find yourself in a jam or need any sort of help our support team is ready 247 to assist you.
Electronic data capture certification. Accelerate Study Cycle Times. As a result doctors researchers and patients are trapped in an inefficient system that leads to data quality issues increasing compliance risk. Electronic Data Capture EDC Our user-friendly EDC interface makes it possible for you to build your entire study the way you want it.
Electronic Data Capture Fundamentals. It has many features including. Electronic data capture systems have become a very popular tool to use in managing data in clinical trials.
See What Makes Our EDC Unique Electronic Patient-Repo. Data Exports Logging User Rights Project Creation 1203. EDC replaces the traditional paper-based data collection methodology to streamline data collection and expedite the time to market for drugs and medical devices.
Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. Run the Study You Want. At OpenClinica we are immersed in.
By using our powerful and intuitive Electronic Data Capture EDC tool youll be able to streamline your data maximize efficiencies tighten up your costs and reach your end goal sooner. Availability Software is available at no cost for REDCap. Introduction to Electronic Data Capture EDC 1747.
Translate complex protocols into elegant casebook. Annonce Electronic Data Capture thats tailor-made for MedTech studies. Annonce Electronic Data Capture thats tailor-made for MedTech studies.
REDCap Research Electronic Data Capture is a secure web application for building and managing online surveys and databases. Its intuitive design means that you dont need a programmer for every small or mid-study change. Electronic Data Capture EDC is the medical abbreviation is admittedly a fairly generic sounding term but in the clinical trials field it actually means something fairly specific.
TrialKit a cloud-based platform available via a web interface and downloadable native mobile app enables electronic data capture EDC and end-to-end clinical trial management for pharmaceutical biotechnology and medical device companies of all sizes. Central to this problem is the swivel chair interoperability requiring manual re-entry of data from source Electronic Health Record EHR systems into the study database electronic data capture EDC system. Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing.
The process reduces data errors to provide researchers with improved data. Some systems have built-in metrics reporting that offer insights into the progress of a study. De très nombreux exemples de phrases traduites contenant electronic data capture system Dictionnaire français-anglais et moteur de recherche de traductions françaises.
An electronic data capture EDC system is a computerized system designed for the collection of clinical data in electronic format for use mainly in human clinical trials. Level up your career in data capturing structural assessments and lifecycle analysis. Electronic Data Capture for clinical investigations and post market clinical follow-up.
An EDC system uses technology to streamline the collection and transmission of clinical trial data from the patient to the research laboratory. Electronic Data Capture for Modern Clinical Trials. And the sooner you wrap up a successful trial the closer we all are to a healthier future.
Eliminate constraints and run the trial you want with a modern EDC system. Key features include WYSIWYG form editor risk-based monitoring activity d. Conversely automations and.
An EDC system. All clinical data lives there and everyone who needs it can access it when and how they want to. Electronic data capture EDC is the computerized collection and management of clinical trial data from patients and subjects.
Once youre ready to pull the data out of the system there are easily accessible exports to extract patient data. Level up your career in data capturing structural assessments and lifecycle analysis. Using systems to collect clinical trial data in electronic form as opposed to paper form.
Electronic Data Capture for clinical investigations and post market clinical follow-up. Online or offline project design Online using the Online Designer or offline using a data dictionary template in Microsoft Excel that can be uploaded later into REDCap. Vault EDC for clinical trials provides a fast and intuitive interface for capturing and reviewing data from sites.
To learn more about the course we invite you to follow the information session available here. Captivate EDC is a cloud-based electronic data capture solution that assists medical researchers and practitioners with data capture and forms management. Deploy studies in weeks not months.
Selecting and Implementing Electronic Data Capture EDC1 August 2 - August 28 2021. Data Imports Scheduling Reports.
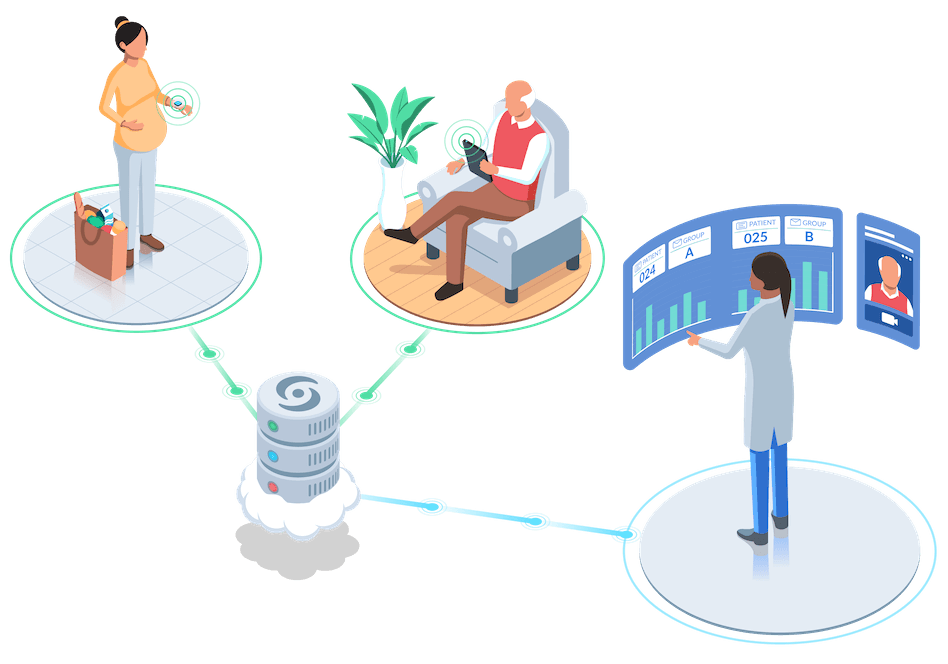
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Qualification Opinion Esource Direct Data Capture Ddc En Pdf

What Is Ecoa And How Does It Improve Clinical Trial Data Quality
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Qualification Opinion Esource Direct Data Capture Ddc En Pdf
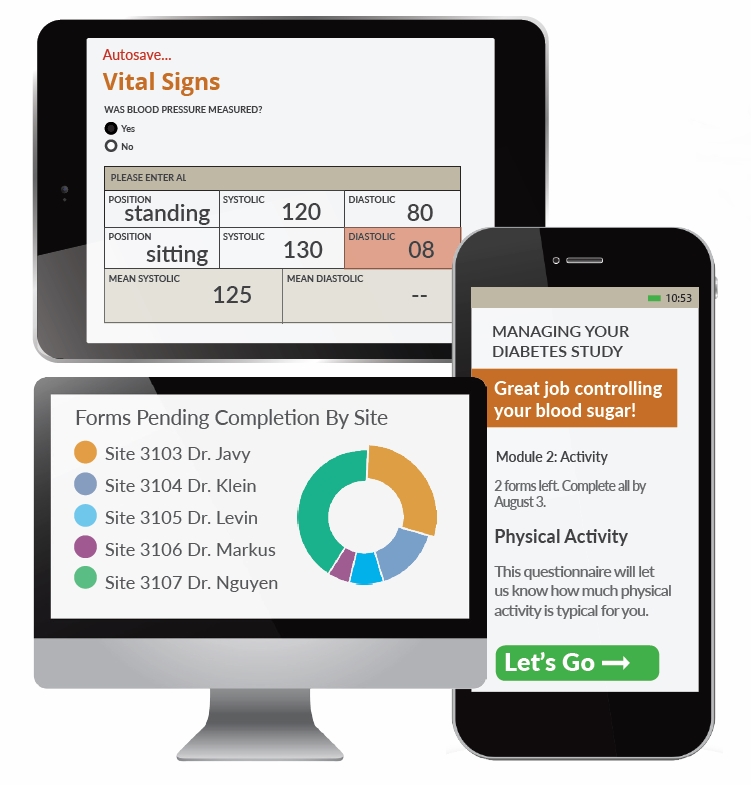
Electronic Data Capture The Fundamentals Of Edc Openclinica

Inform Electronic Data Capture Edc Data Management System
Verify A Signature In Utility Mode Communaute Chorus Pro
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
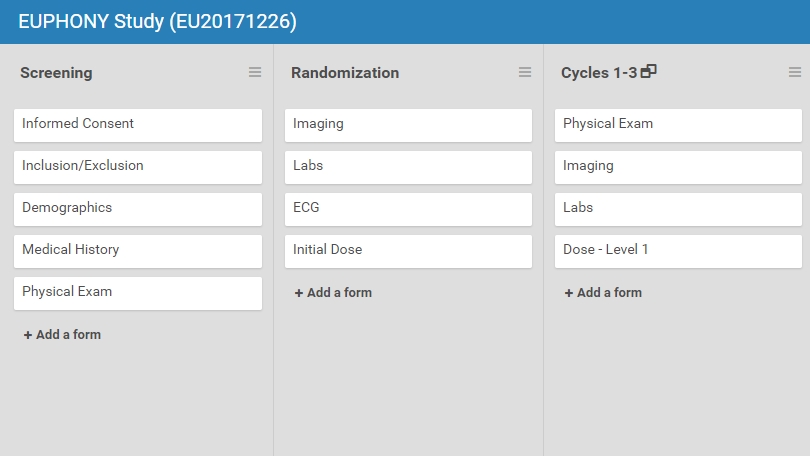
Openclinica Enterprise Edc System Clinical Data Management
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Qualification Opinion Esource Direct Data Capture Ddc En Pdf
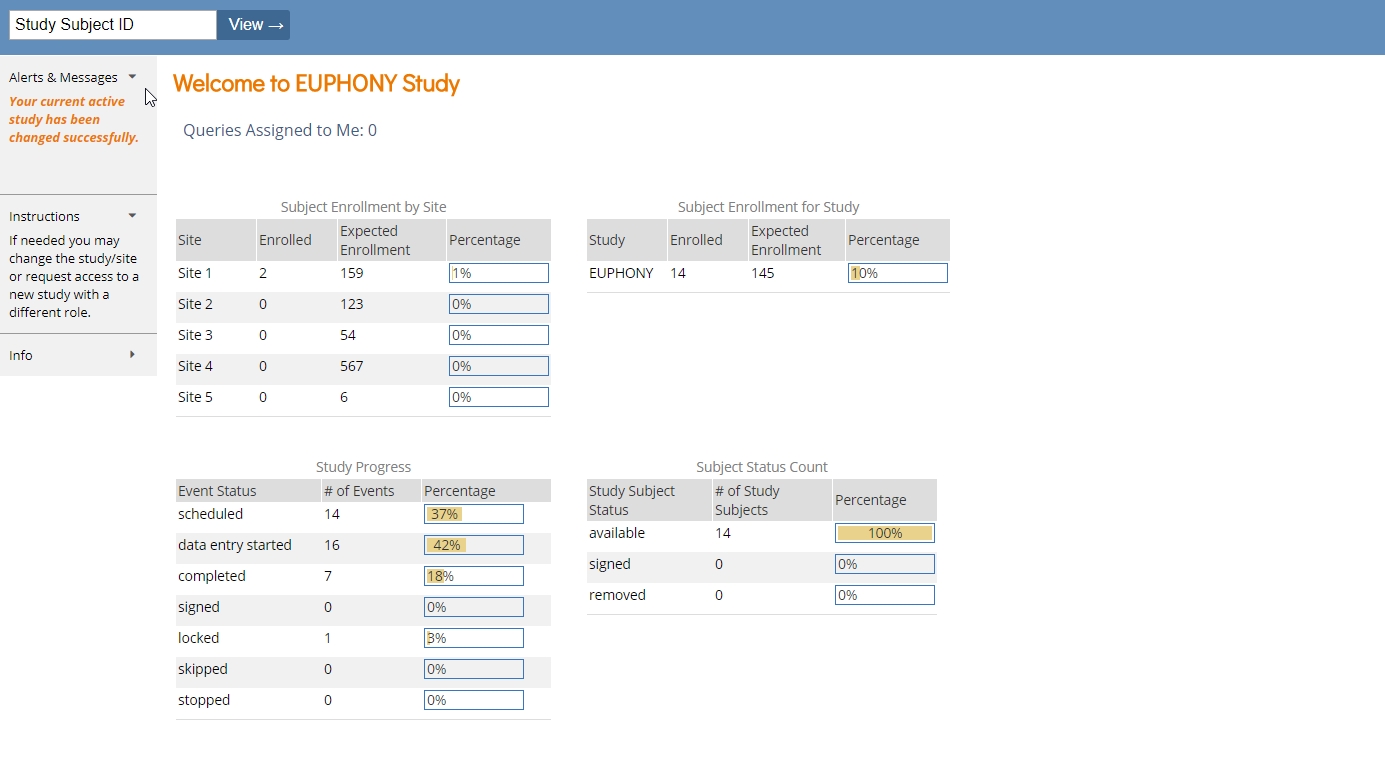
Openclinica Enterprise Edc System Clinical Data Management
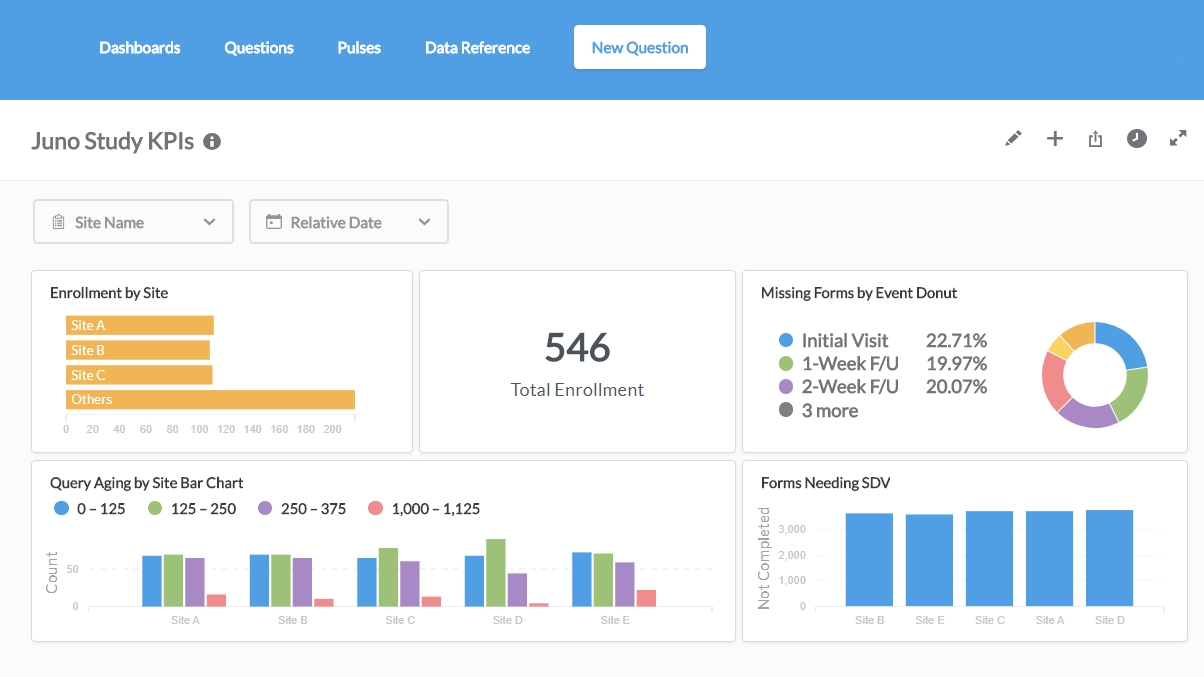
Openclinica Enterprise Edc System Clinical Data Management
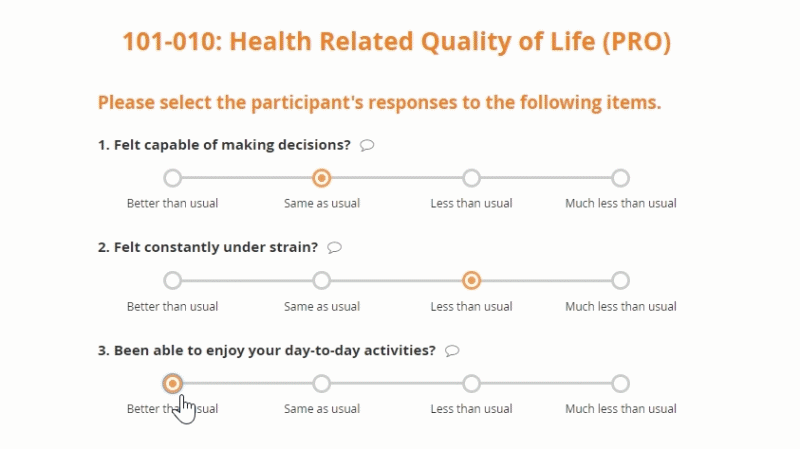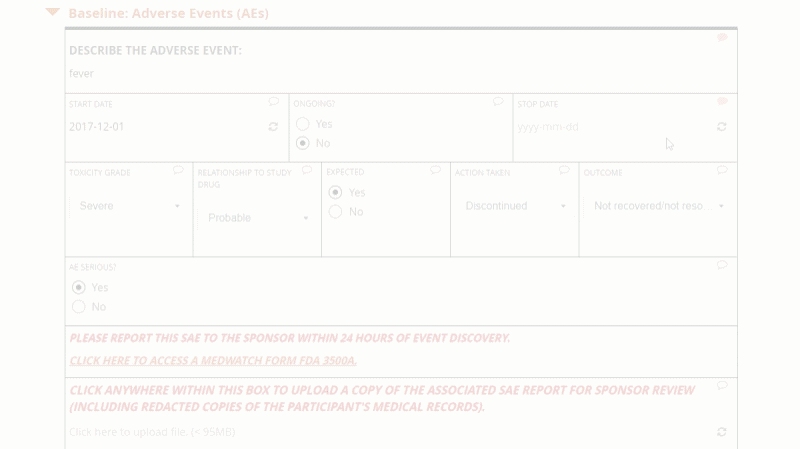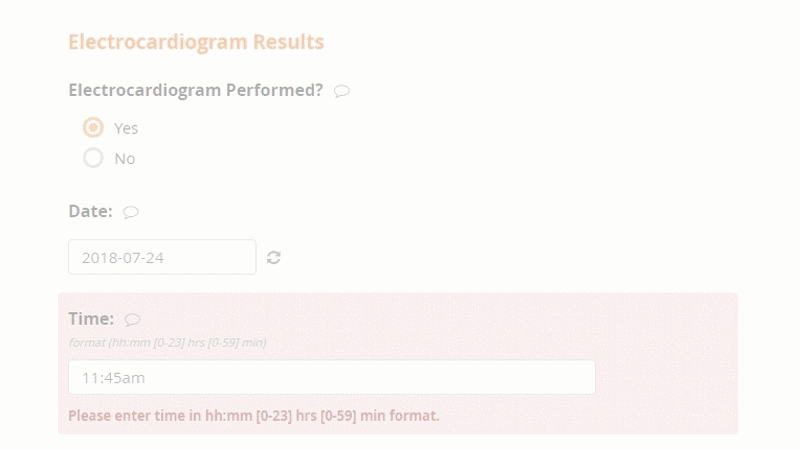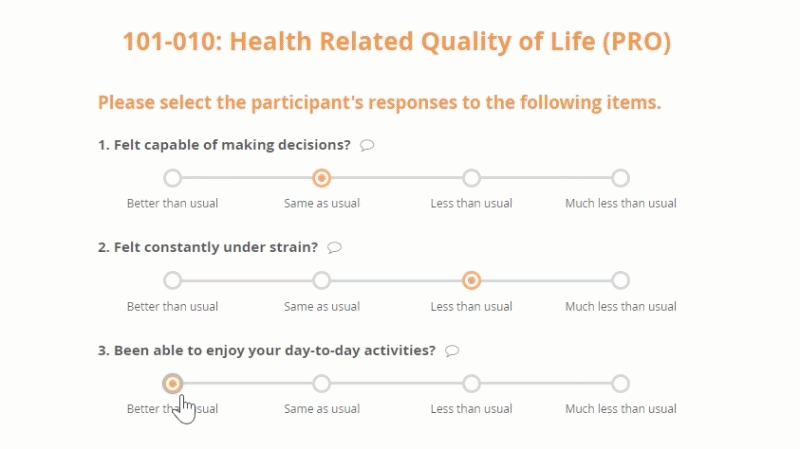
Openclinica Enterprise Edc System Clinical Data Management

Edc Electronic Data Capture Software Ennov Software For Life
Inform Electronic Data Capture Edc Data Management System
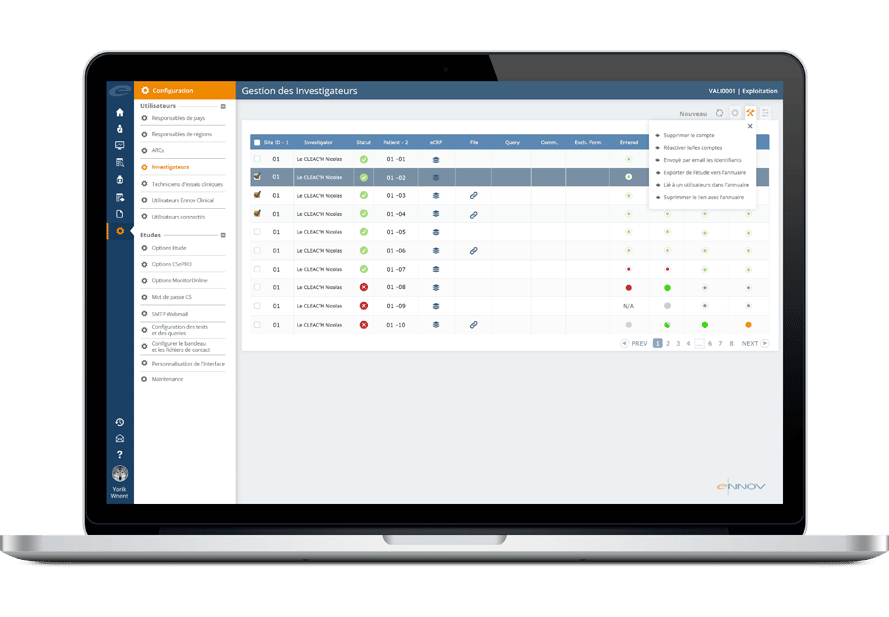
Edc Electronic Data Capture Software Ennov Software For Life
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor
Https Www Ema Europa Eu En Documents Comments Overview Comments Draft Qualification Opinion Esource Direct Data Capture Ddc En Pdf

Post a Comment for "Electronic Data Capture Certification"